Taiyuan Lexus RX new energy price reduction information, the highest profit 80,000! Today’s great benefits
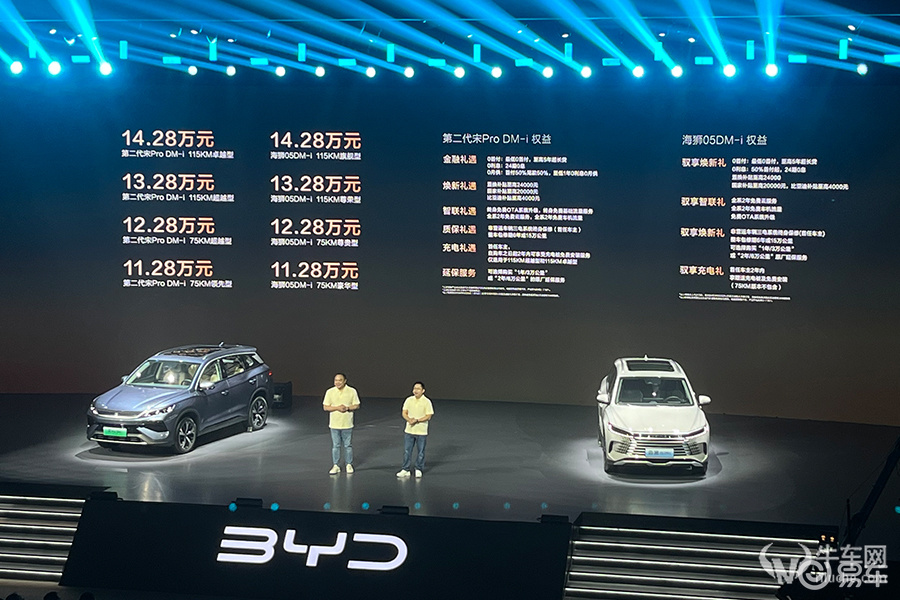
On [Autohome Taiyuan Promotion Channel], we bring you an exciting news. We are giving back to consumers with practical actions. We are currently conducting an unprecedented promotion in Taiyuan. The maximum discount is as high as 80,000 yuan, which brings the starting price of this luxury new energy model down to 469,000 yuan. For consumers who are interested in buying Lexus RX new energy, this is a great opportunity not to be missed. Click "Check the car price" in the quotation form to hurry up and get this real offer in a limited time.

With its unique design concept and exquisite craftsmanship, Lexus RX New Energy shows a unique appearance charm. The front face design adopts the iconic spindle grille of the Lexus family, and is matched with a sharp headlight group, showing the perfect fusion of power and technology. The body lines are smooth, and the overall style combines luxury and movement, highlighting the futuristic feeling of the new energy model. The details are full of ingenuity.

The Lexus RX New Energy exudes a unique side charm with its elegant body proportions. The body measures 4890mm long, 1920mm wide and 1695mm high, and the wheelbase reaches 2850mm, ensuring the comfort of the interior space. The smooth lines on the side of the car reflect the always exquisite design of the Lexus brand. The tire specifications are 235/50 R21 on the front and 235/50 R21 on the back. This configuration not only enhances the driving stability, but also further highlights the delicate and dynamic appearance of its appearance.

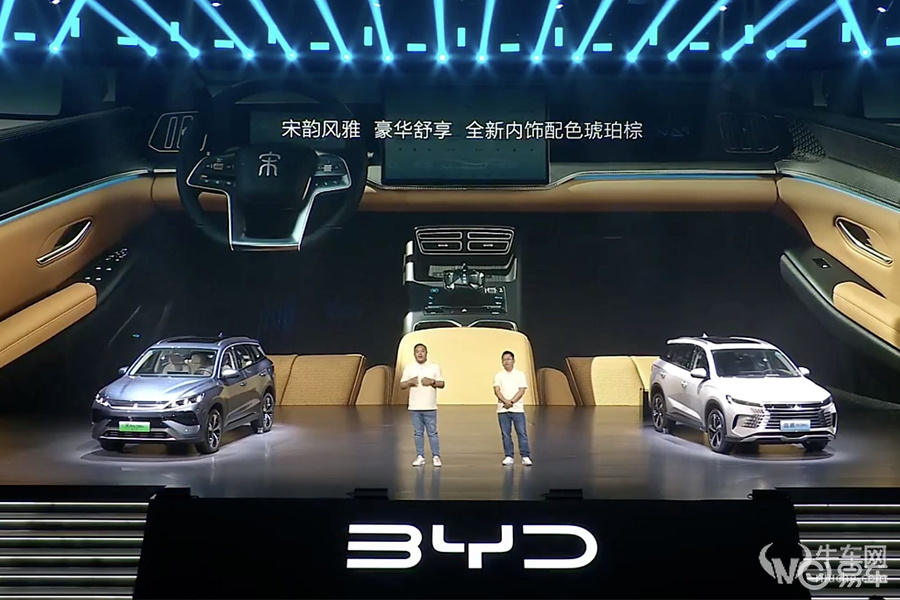
The Lexus RX new energy interior design is based on the concept of luxury and comfort, and the carefully crafted driving space creates an atmosphere where elegance and technology coexist. The steering wheel is made of leather with excellent texture, which not only feels comfortable to hold, but also supports electric up and down + front and rear adjustment to adapt to different drivers’ preferences. The 14-inch central control screen stands in front of the driver and integrates multimedia systems, navigation, telephone and air conditioning functions to easily and quickly meet daily needs.
The seats, whether in the driver’s seat or the passenger’s seat, are equipped with leather materials for advanced comfort. The seats support front and rear, backrest, high and low multi-directional adjustment, and have waist support function to ensure the comfort of long-distance driving. At the same time, the front seats are also equipped with heating and ventilation functions, as well as power seat memory, reflecting Lexus’ attention to detail. The rear seats support backrest adjustment and can be proportionally reclined to provide flexible space options for passengers and cargo. The overall interior design reflects the perfect balance between luxury and practicality found by Lexus RX New Energy.

The car series Lexus RX New Energy is equipped with a 2.5L displacement four-cylinder engine with a maximum power of 136 kW and an output torque of 228 Nm. This power source is equipped with an E-CVT continuously variable transmission, which ensures the smoothness and high efficiency of the vehicle during driving.
To sum up, the Autohome owner expressed high admiration for the appearance of the Lexus RX new energy, especially the front face design, thinking that it exudes a unique high-end temperament. However, he has reservations about the tail design and believes that it needs to be improved. Although there are some minor flaws, overall, the owner’s evaluation of the appearance of the RX new energy is still positive.





















































 In terms of power, the model is equipped with a 4.0T turbocharged 8-cylinder engine as standard. Under the current displacement limitations, this displacement performance has been able to rank among the top. The matching 9AT gearbox can accelerate the model’s 100 kilometers to 4.9 seconds, demonstrating relatively strong strength.
In terms of power, the model is equipped with a 4.0T turbocharged 8-cylinder engine as standard. Under the current displacement limitations, this displacement performance has been able to rank among the top. The matching 9AT gearbox can accelerate the model’s 100 kilometers to 4.9 seconds, demonstrating relatively strong strength.







