What is the progress of BCMA target development? Which biotech will win?
foreword
Malignant multiple myeloma(multiple myeloma ; MM) is a kind ofwithMalignant disease characterized by massive proliferation of monoclonal plasma cells in bone marrow. B cell maturation antigen (BCMA) is highly expressed on the surface of MM cells. In the past few years, great progress has been made in various BCMA targeted immunotherapy for recurrent/refractory MM patients, including anti-BCMA monoclonal antibodies, antibody-drug conjugates (ADC), bispecific T cell binders (BITE) and chimeric antigen receptor adoptive T cell therapy (CAR-T). The 63rd annual meeting of American Hematology Society updated some information about the application of BCMA in MM, and based on this annual meeting, the related contents of this paper were sorted out [1].
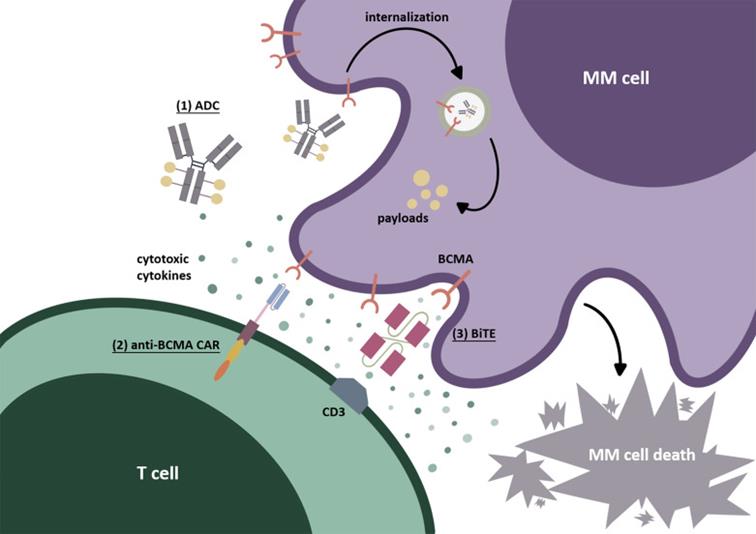
Immunotherapy targeting BCMA: (1)ADC; (2)CAR-T; (3)BiTE;
copywriter | NING
BCell maturation antigen (BCMA)
Also known as TNFRSF17, it is a member of TNF-receptor family, and is called tumor necrosis factor receptor superfamily 17. This receptor is expressed on the surface of B lymphocytes and plasma cells, and is a marker protein of B lymphocyte maturation. Under physiological conditions, BCMA is mainly expressed in plasma mother cells and terminally differentiated plasma cells (PC). Under pathological conditions,BCMA Almost in all MM Tumor cell line (80%–100%) are expressed.The number of BCMA on the surface of malignant PC is much higher than that of conventional PC, which explains why BCMA target has become a battleground for major biological enterprises.

Monoclonal antibody targeting BCMA
SEA-BCMAIt is a new humanized non-fucosylation. IgG1 mAb. The working mechanism of SEA-BCMA may include blocking BCMA activation and its downstream proliferation signal pathway, regulating antibody-dependent cell phagocytosis and enhancing antibody-dependent cytotoxicity.
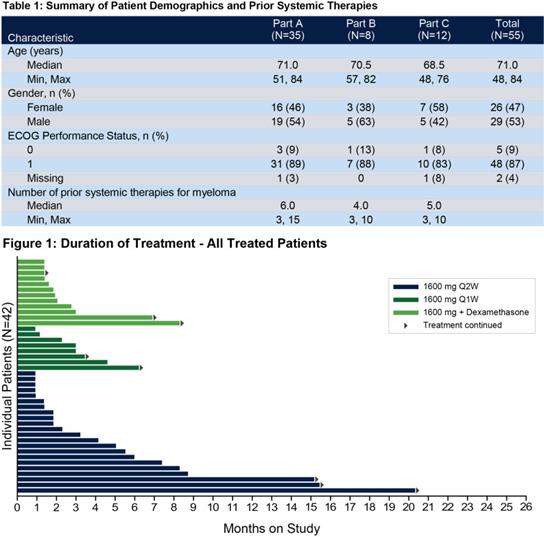
Its clinical phase I trial (SGNBCMA-001; The preliminary results of NCT03582033) show that it has good safety and combination potential: SEA-BCMA also shows encouraging treatment duration and initial anti-tumor activity in the advanced R/R MM population who have received a lot of treatment before.
Source: blood (2021) 138 (Supplement1): 2740.doi: 10.1182/blood-2021-146047.

Bispecific T Cell cement (BITE)
BiTEs can simultaneously target BCMA on MM tumor cells and CD3ε domain of TCR on T cells. After T cells are combined with myeloma cells, cytotoxic T cells can be activated and secrete cytotoxic factors, thus producing cell killing effect.This part is still in Ⅰ at present./Phase ii.
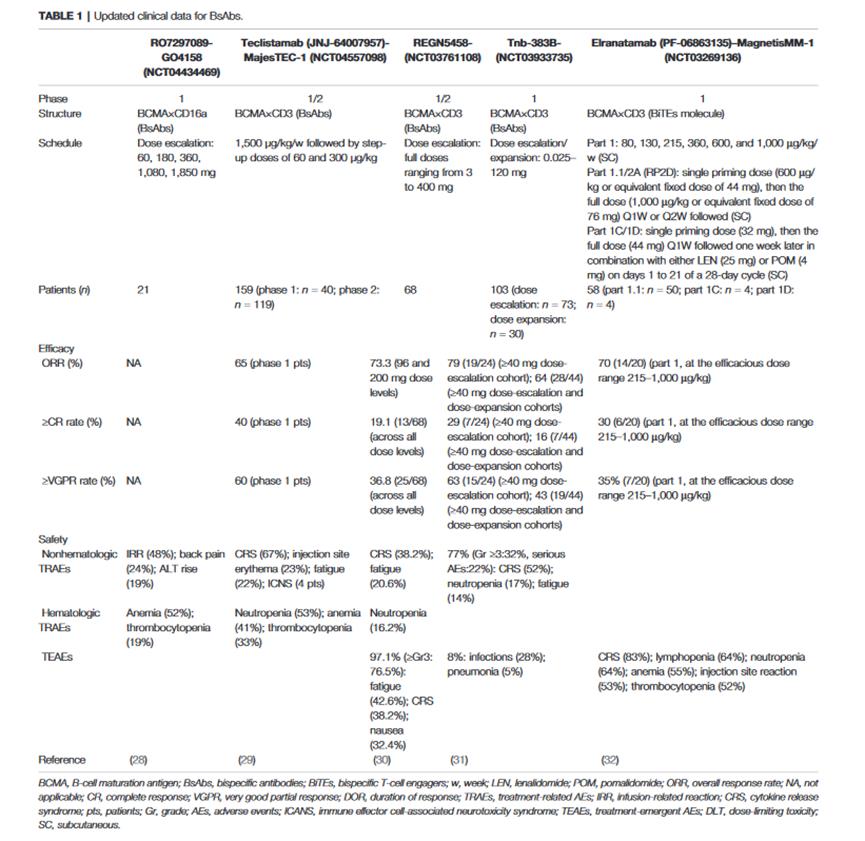
The following table summarizes the clinical data of bispecific antibodies against BCMA, among which the BiTE bispecific type of BCMA×CD3 is the most common.

Novel trispecific antibody drugs
Researchers are no longer satisfied with treating patients with R/R MM through dual-target therapy.HPN217, an extended half-life (median serum half-life:74 Hours) three specificity. T Cell activation construct (TriTAC)It simultaneously targets BCMA and CD3ε (redirected T cells), and adds serum albumin to prolong the half-life, which can exert cytotoxic effect on myeloma cells.Currently working onR/R MM of 1 /2 Phase I clinical trial for evaluation (NCT04184050). Here are some preclinical data.
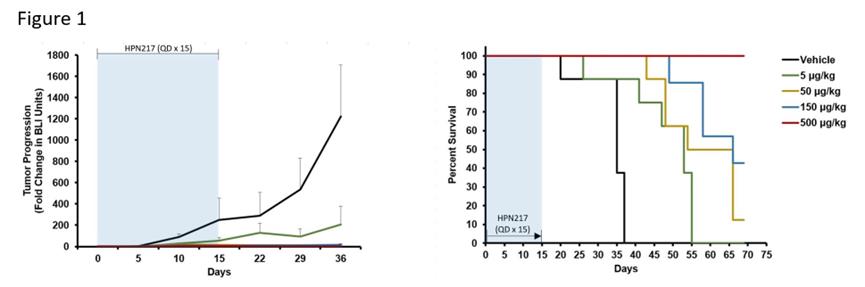
Legend: HPN217 mediates the killing effect of autologous T cells of NCG mice on BCMA-dependent/primary MM cells.
Source: blood (2021) 138 (Supplement1): 1185.doi: 10.1182/blood-2021-151880.
CDR101(CDR-lifeCompany’s products)Is simultaneous targeting.CD3、BCMAandPD-L1The trispecific antibody,T cells can be induced into tumor cells expressing BCMA, and the immunosuppressive effect caused by the interaction between PD-L1 and PD-1 can be blocked at the immune synaptic site. Compared with BCMA × CD3 bispecific drugs, CDR101 leads to at least a tenfold increase in T cell-mediated tumor cell lysis, and performs better than the combination of PD-L1 inhibitor and BCMA × CD3 bispecific drugs.CDR101 It may become a new ready-made treatment, providing a more lasting response and a higher cure rate. CDR101 Has excellent drug-like characteristics, high stability and high stability.CHOHigh yield of expression.Based on these findings, new trispecific immunotherapy drugs have high clinical potential and promising clinical application [2].

Antibody drug conjugate (ADC)
After recognizing BCMA on the cell surface, ADC internalizes it into myeloma cells. Through the degradation of lysosomes or endosomes, the payload is released, causing cytotoxicity.
Belantamab mafodotin (GSK2857916)Is targeted by humanization.BCMA IgG1 andMMAF(tubulin polymerization inhibitor). Belantamab mafodotin was approved by the US Food and Drug Administration (FDA) in 2020 to treat patients with R/R MM.This is the first target approved for listing.BCMAimmunotherapy,A multicenter phase II clinical study (NCT03525678) confirmed that the recommended regimen for patients was 2.5 mg/kg, once every three weeks, but the toxicity of the drug was mainly manifested in thrombocytopenia and pericorneal lesions, such as microcystic epithelial changes or superficial punctate keratopathy. In addition to belantamab mafodotin, several other ADCs targeting BCMA, such as AMG 224, MEDI2228 and HDP-101, have also conducted many preclinical or clinical studies at different stages.
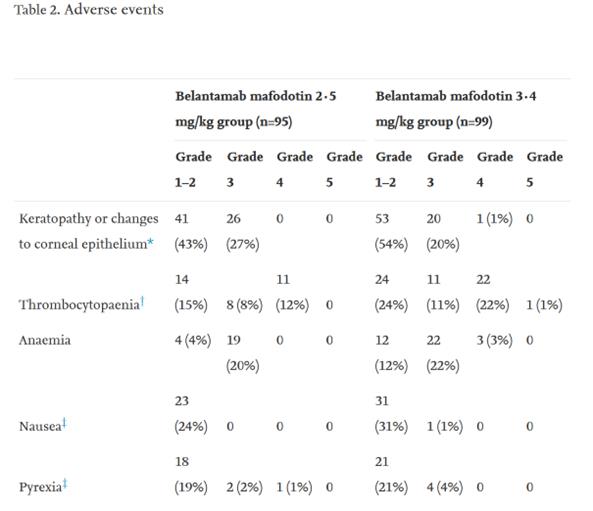
Legend: Incidence rate of adverse events caused by Belantamab mafodotin at 2.5 mg/kg and Q3W.

Antibody drug conjugate (ADC) joint test.
In the joint experiment, there are two schemes: belantamab+DEX (dexamethasone) and belantamab+DEX+POM.
belantamab(2.5 mg/kg Q3W) and DEX(20-40 mg Q1W,threeCycle) jointAfter use, the objective remission rate (ORR) was 46%, the complete remission rate (CR) was 14%, the median progression-free survival (PFS) was 4.9 months, and the median overall survival (OS) was 7.4 months. The incidence of AEs from high to low is anemia (83%), keratopathy (82%; Gr3/4: 56%), thrombocytopenia (70%) and so on.
belantamab+DEX+POMUnion ofIn the experimental scheme, the ORR was 88.9%(48/54), and the CR and PR rates were 24.1%(13/54) and 20.4%( 11/54) respectively. However, keratopathy (96.9%) was also the most common AE, and 56.7% of such patients had reached Gr 3/4. These two studies show that POM and DEX may have a positive effect on the curative effect of belantamab. howeverKeratosis is still a challenge in the treatment process.
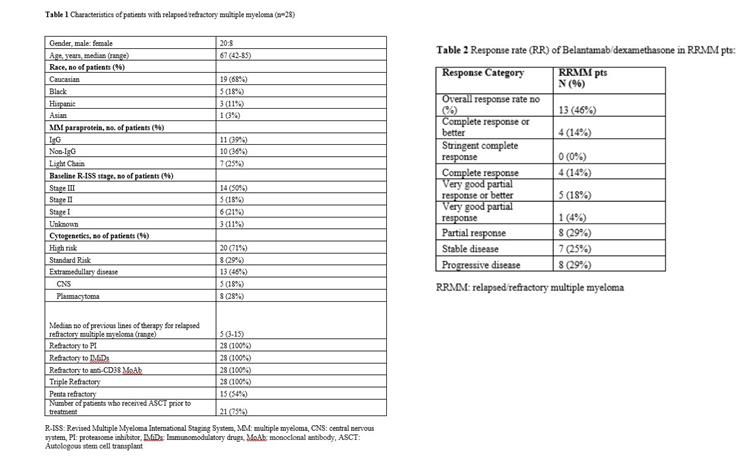
Legend: Detailed rules and results of belantamab+DEX (Dexamethasone) joint trial scheme.
Source: Blood 2021; 138 (Supplement 1): 1642. doi: https://doi.org/10.1182/blood-2021-149791

CAR-Ttherapy
nowTwo CAR-T therapies based on BCMA have been approved for marketing.And is used for treating patients with multiple myeloma.
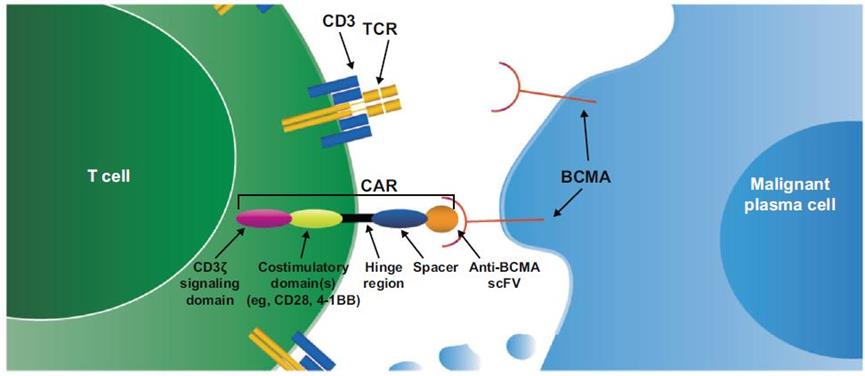
Source: "CAR-T therapy targeting BCMA"
7.1
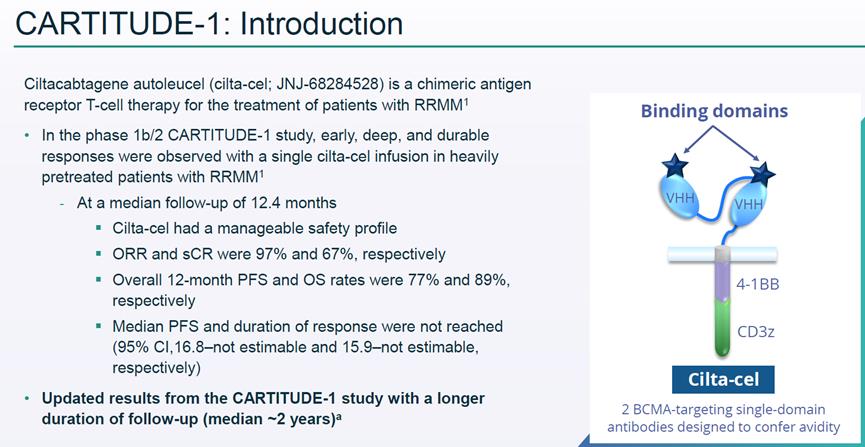
Cilta-cel (Carvykti) for Orense injection.
Sidaki Orense injection(Cilta-cel,Carvykti) yes2022year2moon28Listed in China (NMPAApproved for listing/FDAFormal approval) targeting.BCMAofCAR-TCell products, jointly developed by Janssen and Legendary Bio, Legendary Bio owns 70% interests in Carvykti Greater China and 50% interests overseas.
In CARTITUDE-1 trial, the overall remission rate was 97%, the 18-month progression-free survival rate was 66%, and the 2-year progression-free survival rate was 61%. The 18-month overall survival rate is 81%, and the 2-year overall survival rate is 74%. Compared with similar products, this data is very gratifying. According to the latest news from Kingsley, the sales of the legendary BCMA CAR-T new drug Carvykti in the first half of the year was 24 million US dollars.

7.2
Abecma(Ide-cel)
2021yearthreemoon26Day,FDAapproveBaishimei SquibbAbecma(Ide-cel)be listedAbecma is also a BCMA-oriented CAR-T cell therapy, which is customized from patients’ autologous T cells. Clinical data show that the overall remission rate of all patients is 72%, of which the complete remission rate is 28%; Even for patients who have received various regimens, the remission rate of Abecma still reaches 72%, and this remission is very lasting, and 65% of patients are over 12 months.
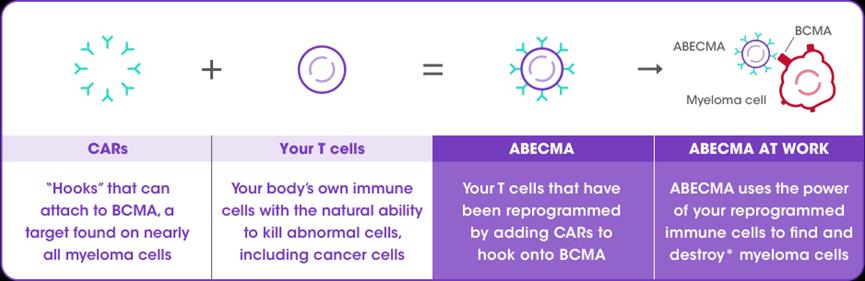
Legend: preparation principle of Abecma
7.3
Iqalunsei injection
In addition, June 2, 2022Reindeer biology/Xinda biologyAnnounce BCMA CAR-T therapyIqalunsei injectionThe listing application was obtained.NMPAAccepted, it is expected to become the first domestic independent research and development of the whole process.CAR-Ttherapy. The clinical research data reported at the 63rd ASH annual meeting in 2021 showed that the overall remission rate (ORR) was 94.9%, and the complete remission rate/strict complete remission rate (CR/sCR) was 58.2%, showing the excellent safety and effectiveness of Iqalunsei injection.
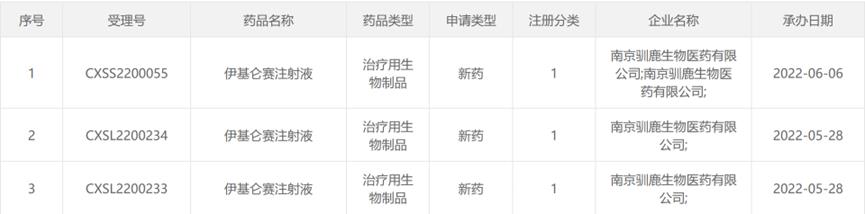
Source: NMPA official website
7.4
The clinical trial of CAR-T therapy targeting BCMA is as follows
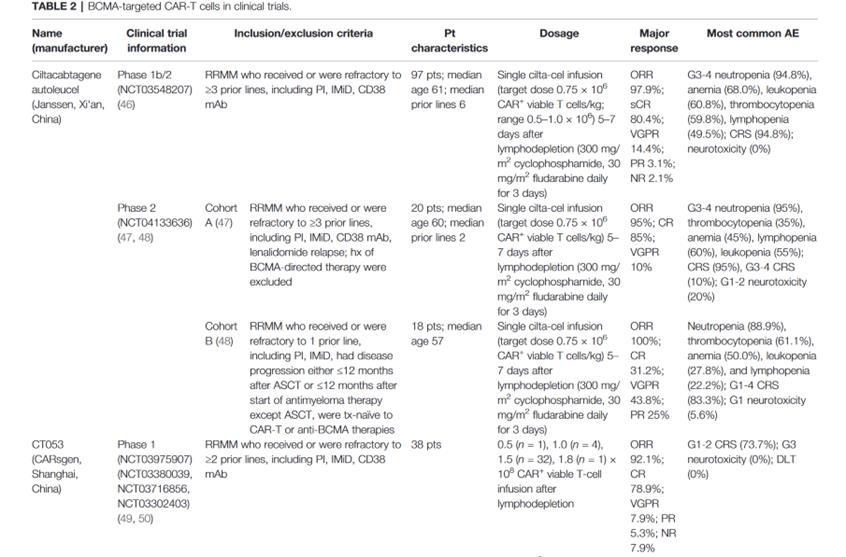

summary
Judging from the listing situation,Belantamab mafodotin (GSK2857916) is composed of humanized targeting BCMA IgG1 and MMAF (tubulin polymerization inhibitor), which is the first targeted BCMA immunotherapy approved for marketing. At present, there are two CAR-T therapies that have been put on the market, developed by Bristol-Myers Squibb respectively.Abecma(Ide-cel), developed in cooperation with Janssen)/ Legendary Biology.Sidaki Orense injectionFrom the data point of view, the latter effect is better, the ORR is increased from 72% to 97%, and other indicators are relatively better. On June 2nd, 2022, Reindeer Bio/Cinda Bio announced BCMA CAR-T therapy.Application for marketing of Iqalunsei injectionIt has also been accepted by NMPA, and it is expected to become the first CAR-T therapy independently developed in the whole process in China. Let us wait and see! From the therapeutic point of view, CAR-T therapy is at the forefront in terms of clinical data and market situation, but we can’t simply judge the advantages and disadvantages of these products. For example, better anti-BCMA CAR-T cell therapy requires more complicated preparation conditions and more expensive treatment costs, which are unbearable for ordinary families. Therefore, while continuing the existing research, it is necessary to study the potential mechanism affecting the curative effect in order to optimize BCMA targeted immunotherapy.
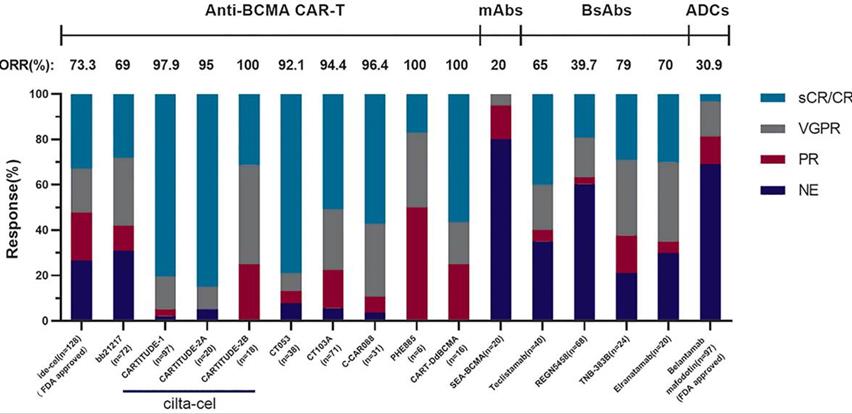
Legend: Comparison of clinical efficacy between different immunotherapy products targeting BCMA (see [1] for specific clinical data).
reference data
[1]Targeting BCMA to Treat Multiple Myeloma: Updates From the 2021 ASH Annual Meeting. Front Immunol. 2022 Mar 7; 13:839097. doi: 10.3389/fimmu.2022.839097. PMID: 35320942; PMCID: PMC8936073.
[2] Blood 2021; 138 (Supplement 1): 1583. doi: https://doi.org/10.1182/blood-2021-152160
[3] Blood 2021; 138 (Supplement 1): 1642. doi: https://doi.org/10.1182/blood-2021-149791
* Tweets are used to transfer knowledge. If you have any questions about copyright, please contact BiG Bio-innovation Society within 30 days after the publication of this article.